
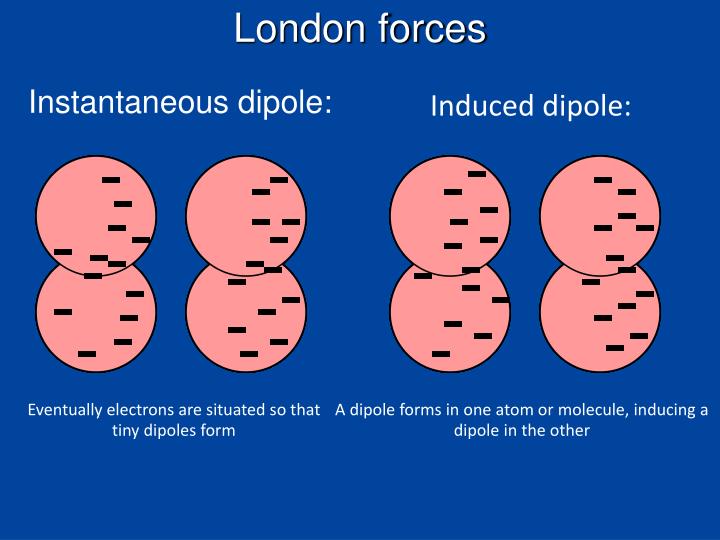

Those temporary dipoles will actually induce dipoles in neighboring molecules. Let’s look at what happens when there are more dibromide molecules in the area.

Of course, molecules don’t exist in isolation. Those partial charges will oscillate as electron density moves around those partial positive charges result from lack of electron density. The two atoms in the molecule are identical, so it might seem at first that the electrons will have a symmetrical distribution, but that’s an oversimplification.Įlectron density will actually oscillate-this happens because electrons aren’t point-objects it might help to think about them as clouds of probability. Let’s say we’ve got a single dibromide molecule like the one above. Temporary polarity resulting from unsymmetrical distribution of electrons creates an attractive force in otherwise nonpolar molecules like Br2. Alkanes are non-polar and exhibit no hydrogen bonding, so Van der Waals London dispersion are the only IMFs they exhibit. Real-life examples include gasoline and petroleum jelly, which are mostly make of alkanes of varying lengths. London dispersion forces are actually what hold many substances together. London dispersion forces are the weakest of the three types of intermolecular forces. London dispersion is most easily noticed in nonpolar molecules, where stronger IMFs don’t drown out the London dispersion. Intermolecular forces directly determine properties like melting point, boiling point, and viscosity. London dispersion forces and dipole-dipole forces are subsets of Van der Walls forces, which themselves are the weakest intermolecular forces.


 0 kommentar(er)
0 kommentar(er)
